This video will guide you through the steps for the preparation and storage of NutriHope® Fibre.
Instructions for safe preparation and storage of NutriHope® Fibre at home (printable version can be downloaded below)
Clean the surface and utensils to be used. Wash your hands.
Boil safe drinking water.
Pour into a clean glass or a bigger container if easier to mix the required amount of boiled water. Wait until the water cools to 60-70°C (approx. 5 min) for an optimal reconstitution.
In the glass or container, add the prescribed number of levelled scoops of powder.
| Number of levelled scoops |
Volume of water (ml) |
kcal | Proteins (g) |
| 3 | 200 | 300 | 15 |
| 4 | 250 | 400 | 20 |
Note that a standard glass contains 200-250 ml. If the correct volume of water cannot be measured precisely, exceeding these advised volumes will not give rise to medical consequences unless you are a patient subject to fluid intake restrictions.
Place the dry scoop immediately back into the tin, do not wash. Close the tin.
Mix thoroughly until dissolved completely. Stir with a clean fork or a wire whisk for an optimal reconstitution. Cool to feeding temperature depending on preference.
Use immediately after preparation: once reconstituted, use within 2 hours. If the product is not consumed immediately, cover.
The formula reconstituted for less than 2 hours can be stored in a refrigerator (5 °C max.) for a maximum of 24 hours. Beyond 24 hours, the refrigerated formula should be discarded. When used after refrigeration, stir the formula before consuming.
The opened tin should be stored in a cool, dry place away from direct sunlight. Once opened, use within 4 weeks.
Why use NutriHope® Fibre?
Malnutrition is a major risk factor associated with high mortality and morbidity, functional decline, prolonged hospital stays, and increased health care costs. This situation is reflected the world over. A systematic review evaluated the prevalence of undernutrition in 61% of adults undergoing surgery in low- and middle-income countries. A prospective cohort study including adult patients from hospitals in South Africa, Kenya, and Ghana revealed that nearly two-thirds of patients were at risk of malnutrition at admission. This was associated with longer length of stay and higher hospital mortality.
Nutritional support, when provided during the hospital stay, may offset some of these adverse outcomes. For this reason, international organisations recommend screening patients for malnutrition risk and using nutritional support for patients at nutritional risk or who are malnourished. Enteral nutrition is a vital component of nutrition therapy for patients with malnutrition. It allows for delivery of nutrients to those who cannot maintain adequate nutrition by oral intake alone. Nutritional support has been demonstrated to decrease mortality, with a more pronounced effect in patients with established malnutrition, to reduce nonelective hospital readmissions, to increase energy and protein intake and weight. The 30-day readmission rate was also lower for patients classed as suffering from malnutrition who received nutritional support than for those who did not benefit from such support.
NutriHope® Fibre is a complete hyperproteic nutritional powder intended for the dietary management of adult patients with or at risk of disease related malnutrition. Once reconstituted it can be used for enteral tube and/or oral feeding for a variety of diseases and conditions where nutritional support is warranted, for exclusive or partial feeding.
References:
- Jones, D. et al. Malnutrition and nutritional screening in patients undergoing surgery in low and middle income countries: A systematic review. JCSM Clin. Rep. 7, 79–92 (2022).
- Blaauw, R. et al. The Problem of Hospital Malnutrition in the African Continent. Nutrients 11, 2028 (2019).
- Kondrup, J. ESPEN Guidelines for Nutrition Screening 2002. Clin. Nutr. 22, 415–421 (2003).
- Mueller, C., Compher, C., Ellen, D. M., & the American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors. A.S.P.E.N. Clinical Guidelines: Nutrition Screening, Assessment, and Intervention in Adults. J. Parenter. Enter. Nutr. 35, 16–24 (2011).
- Gomes, F. et al. Association of Nutritional Support With Clinical Outcomes Among Medical Inpatients Who Are Malnourished or at Nutritional Risk: An Updated Systematic Review and Meta-analysis. JAMA Netw. Open 2, e1915138 (2019).
- Kaegi-Braun, N., Mueller, M., Schuetz, P., Mueller, B. & Kutz, A. Evaluation of Nutritional Support and In-Hospital Mortality in Patients With Malnutrition. JAMA Netw. Open 4, e2033433 (2021).
Is NutriHope® Fibre milk?
No, NutriHope® Fibre is not a milk powder. NutriHope® Fibre is a complete hyperproteic nutritional powder adapted to adult patients at risk of, or with, disease-related malnutrition. While it contains milk protein, it contains twice more protein than cow’s milk and also contains non-milk ingredients.
To know moreNutriHope® Fibre cannot be referred to as a therapeutic milk. F-75 and F-100, which are referred to as therapeutic milks, are product specifically developed for the nutritional recovery of children suffering from severe acute malnutrition.
Once reconstituted, NutriHope® Fibre is an enteral formula intended for the dietary management of adult patients with or at risk of disease-related malnutrition. It can be used for a large variety of diseases and conditions where nutritional support is warranted, for exclusive or partial feeding of patients whose ability to ingest, digest, absorb or metabolise ordinary foods is limited or impaired, or who, for medical reasons, have other special nutrient requirements. As such, it is a dietary food for special medical purposes (FSMP) and must be used under medical supervision.
What are the contra-indications of NutriHope® Fibre?
- Not adapted for individuals who do not have the diseases, disorders or medical conditions for which the product is intended
- Not suitable for parenteral (I.V.) administration.
- Not suitable for individuals < 13 years old.
- Not suitable for patients with galactosaemia.
- Not suitable when enteral nutrition is not permitted (gastrointestinal obstruction, bowel ischemia or necrosis, etc.).
- Contraindicated if allergy and/or medical condition impede the exposure to any constituent of the product.
- Do not mix drugs or other foodstuffs with the product.
Why are there fibres in NutriHope® Fibre?
For most patients, fibres are recommended in enteral formulas. Fibres are recognized as an essential part of the normal diet. Fibres have many physiological effects which are relevant for enterally-fed patients. Fibres promote regular bowel movement, reduce the risk of constipation, and significantly reduce the incidence of diarrhea. Fibre is safe and generally well-tolerated in patients. Inclusion of a mixture of soluble and insoluble fibres is today considered to represent a more physiological approach.
Why does the water need to be boiled?
Boiling is the safest method of killing pathogenic germs, including viruses, bacteria, and parasites. Bring clean water to the boil for at least 1 minute. There is no need to boil water any longer since, as soon as this temperature is reached, all microorganisms that can affect health are eliminated. Please note: The boiling point of water can vary depending on the altitude.
Why does the water to be mixed with NutriHope® Fibre need to be cooled to at least 70 °C?
It is recommended to dilute NutriHope® Fibre powder in water cooled to at least 70 °C. Above this temperature, the micronutrients in the formula could be affected. Below this temperature, dilution is possible but may take longer and leave residues at the bottom of the container. The dispersion quality also depends on the volume to be prepared, the container and the type of utensil used for reconstitution (whisk, fork).
This video will guide you through the steps for the preparation and storage of NutriHope® Fibre.
Instructions for safe preparation and storage of NutriHope® Fibre at home (printable version can be downloaded below)
Clean and disinfect the work surface, wash your hands and use sterilised utensils and container, if possible.
Boil safe drinking water.
Pour into a container the correct amount of boiled water cooled to 60-70 °C for an optimal reconstitution.
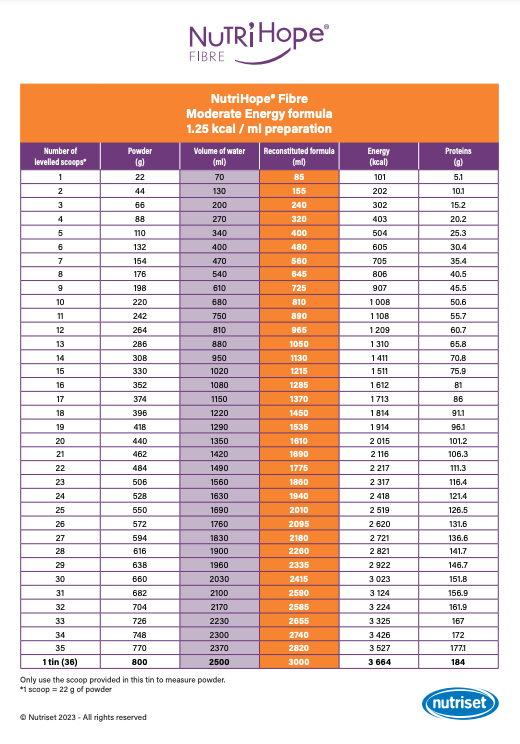
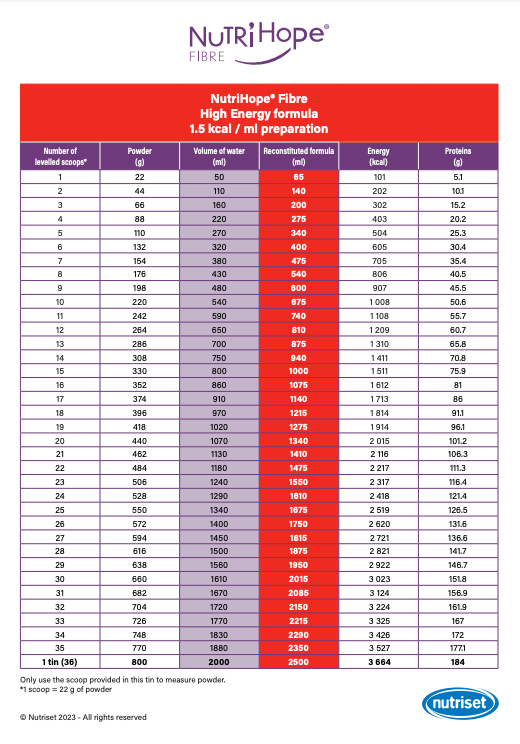
In the container, add the required number of levelled scoops of powder (see “Reconstitution chart“): follow the instructions for Moderate Energy (ME) or High Energy (HE) formulation depending on medical prescription.
Place the dry scoop immediately back into the tin, do not wash.
Mix thoroughly until dissolved completely. Stir with a wire whisk for an optimal reconstitution; for smaller amounts, a clean fork can also be used.
For enteral tube use, pour prescribed amount of final reconstituted ME or HE formula into the patient’s bag, label accordingly and deliver following prescribed regime. For oral use, cool to feeding temperature.

Use preferably instantly after preparation. If the product is not consumed immediately, cover. Once reconstituted, use within 6 hours at room temperature for enteral tube feeding and within 2 hours for oral feeding.
Formula reconstituted for less than 2 hours can be stored in a refrigerator (5 °C max.) for a maximum of 24 hours. Beyond 24 hours, refrigerated formula should be discarded. If used after refrigeration, stir the formula before consuming.
This reconstitution chart will guide you in dosing NutriHope® Fibre according to energy and protein targets.
Why use NutriHope® Fibre?
Malnutrition is a major risk factor associated with high mortality and morbidity, functional decline, prolonged hospital stays, and increased health care costs. This situation is reflected the world over. A systematic review evaluated the prevalence of undernutrition in 61% of adults undergoing surgery in low and middle-income countries. A prospective cohort study including adult patients from hospitals in South Africa, Kenya, and Ghana revealed that nearly two-thirds of patients were at risk of malnutrition at admission. This was associated with longer length of stay and higher hospital mortality.
Nutritional support, when provided during the hospital stay, may offset some of these adverse outcomes. For this reason, international organisations recommend screening patients for malnutrition risk and using nutritional support for patients for patients at risk of, or suffering from, malnutrition. Enteral nutrition is a vital component of nutrition therapy for patients with malnutrition. It allows for delivery of nutrients to those who cannot maintain adequate nutrition by oral intake alone. Nutritional support has been demonstrated to decrease mortality, with a more pronounced effect in patients with established malnutrition, to reduce nonelective hospital readmissions, to increase energy and protein intake and weight. The 30-day readmission rate was also lower for patients classed as suffering from malnutrition who received nutritional support than for those who did not benefit from such support.
NutriHope® Fibre is a complete hyperproteic nutritional powder intended for the dietary management of adult patients with or at risk of disease related malnutrition. Once reconstituted it can be used for enteral tube and/or oral feeding for a variety of diseases and conditions where nutritional support is warranted, for exclusive or partial feeding.
References:
- Jones, D. et al. Malnutrition and nutritional screening in patients undergoing surgery in low and middle income countries: A systematic review. JCSM Clin. Rep. 7, 79–92 (2022).
- Blaauw, R. et al. The Problem of Hospital Malnutrition in the African Continent. Nutrients 11, 2028 (2019).
- Kondrup, J. ESPEN Guidelines for Nutrition Screening 2002. Clin. Nutr. 22, 415–421 (2003).
- Mueller, C., Compher, C., Ellen, D. M., & the American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors. A.S.P.E.N. Clinical Guidelines: Nutrition Screening, Assessment, and Intervention in Adults. J. Parenter. Enter. Nutr. 35, 16–24 (2011).
- Gomes, F. et al. Association of Nutritional Support With Clinical Outcomes Among Medical Inpatients Who Are Malnourished or at Nutritional Risk: An Updated Systematic Review and Meta-analysis. JAMA Netw. Open 2, e1915138 (2019).
- Kaegi-Braun, N., Mueller, M., Schuetz, P., Mueller, B. & Kutz, A. Evaluation of Nutritional Support and In-Hospital Mortality in Patients With Malnutrition. JAMA Netw. Open 4, e2033433 (2021).
Is NutriHope® Fibre milk?
No, NutriHope® Fibre is not a milk powder. NutriHope® Fibre is a complete hyperproteic nutritional powder adapted to adult patients at risk of, or with, disease-related malnutrition. While it contains milk protein, it contains twice as much protein than cow’s milk and also contains non-milk ingredients.
To know moreNutriHope® Fibre cannot be referred to as a therapeutic milk. F-75 and F-100, which are referred to as therapeutic milks, are product specifically developed for the nutritional recovery of children suffering from severe acute malnutrition.
Once reconstituted, NutriHope® Fibre is an enteral formula intended for the dietary management of adult patients with or at risk of disease-related malnutrition. It can be used for a large variety of diseases and conditions where nutritional support is warranted, for exclusive or partial feeding of patients whose ability to ingest, digest, absorb or metabolise ordinary foods is limited or impaired, or who, for medical reasons, have other special nutrient requirements. As such, it is a dietary food for special medical purposes (FSMP) and must be used under medical supervision.
If a patient has or is showing signs of developing diarrhoea, can NutriHope® Fibre be administered?
Yes, NutriHope Fibre can be administered to a patient who has or is showing signs of developing diarrhoea.
To know moreDiarrhoea can occur frequently in hospitalised patients, especially critically ill patients, including those who receive enteral nutrition. The frequency and causes of diarrhoea will vary according to the pathology of the patient, his circumstances (unit in which he is hospitalised; drugs being administered; etc.), and the population to which he belongs to (young, old, place of origin etc.), among other causes. Although multicausal, it is often erroneously associated with the supply of enteral nutrition.
As a general rule, enteral nutrition does not need to be interrupted for diarrhoea and should be continued while the aetiology is being investigated. Generally, diarrhoea is categorized as motility-related, malabsorptive, inflammatory/exudative, secretory, or osmotic. Therefore, managing diarrhoea in the enterally fed patient requires a systematic approach prior to making changes to the enteral feeding regimen. Understanding the type of diarrhoea can help establish a differential diagnosis and guide treatment strategies. Diarrhoea in patients who receive enteral nutrition is often caused by such conditions as diabetes, malabsorption syndromes, infection, gastrointestinal complications, or concomitant drug therapy instead of the enteral formula. Factors related to the enteral nutrition that may contribute to diarrhoea include the composition of the formula, the manner of administration, or bacterial contamination.
To ensure that the nutritional requirements of patients are met, and the appropriate treatment is administered, all possible causes of diarrhoea should be considered to take the best clinical decision for the patient, before reducing or discontinuing the amount of formula delivered. This should be taken on a case-by-case basis, and it is the responsibility of the health care professionals to reach to the correct diagnosis and management of diarrhoea in each patient.
References:
- Kozeniecki M, Fritzshall R. Enteral Nutrition for Adults in the Hospital Setting. Nutr Clin Pract. 2015 Oct;30(5):634-51. doi: 10.1177/0884533615594012. Epub 2015 Jul 22. PMID: 26203073.
- Tutorial on Diarrhea and Enteral Nutrition: A Comprehensive Step-By-Step Approach (review).
- Marina Regueira Pitta MD, Franciele Maciel Campos RD, Alline Gonçalves Monteiro RD, Anna Gabriella Ferreira Cunha RD, Juliana Dourado Porto RN, Raquel Rodrigues Gomes RD First published: 11 August 2019 https://doi.org/10.1002/jpen.1674
- Eisenberg PG. Causes of diarrhea in tube-fed patients: a comprehensive approach to diagnosis and management. Nutr Clin Pract. 1993 Jun;8(3):119-23. doi: 10.1177/0115426593008003119. PMID: 8289759.
Can NutriHope® Fibre be provided to persons with hypertension?
Yes, NutriHope® Fibre can be safely used in patients with hypertension.
To know moreThe daily amount of enteral nutrition is tailored to the nutritional and fluid needs of each patient. Therefore, a patient may receive different amounts of formula, and in a final solution of varying concentration (ie. moderate energy or high energy) depending on the patient’s underlying condition and clinical status.
Most complete enteral formulas are formulated to provide 100 % of the recommended daily vitamin and mineral dose when a minimum of approximately 1000 or more kcals are delivered per day.
NutriHope® Fibre provides 2,7 grams of salt in 1 Litre of Moderate Energy solution (1250 kcal), and 3,3 grams in the High Energy one (1500 kcal). Humans have the capacity to adapt to a wide variety of sodium intake and the ability of humans to do so reflects the capacity of the normal human body to conserve or excrete sodium by reducing losses or increasing excretion to maintain sodium equilibrium.
High blood pressure, also called hypertension, is blood pressure that is higher than normal. Several physiological mechanisms are involved in the maintenance of normal blood pressure, and their disturbance may play a part in the development of hypertension. It is probable that many interrelated factors contribute to the increased blood pressure in hypertensive patients, and their relative roles may differ between individuals. Among the factors that have been studied intensively are salt intake, obesity and insulin resistance, the renin-angiotensin system, and the sympathetic nervous system. Other factors have been evaluated, including genetics, endothelial dysfunction, low birth weight and intrauterine nutrition, and neurovascular abnormalities.
WHO has issued a number of recommendations to prevent hypertension, including reducing salt intake to less than 5g daily. As well, a low-salt diet is recommended in most guidelines as a component of nonpharmacologic therapy of primary hypertension, with different thresholds depending on the issuing organisation (WHO < 2 grams/day; US dietary guidelines < 2,3 grams/day etc).
Treatment of hypertension involves nonpharmacologic therapy (also called lifestyle modification, which includes diet) alone or in concert with antihypertensive drug therapy. In hospitalised patients, hypertension treatment must continue, and drug therapy should be individualized and adjusted depending on medical assessment and clinical decision.
Hypertension is not a contraindication to receive enteral nutrition, rather, all the factors influencing blood pressure levels and sodium balance should be assessed for each patient and therapy adjusted accordingly. NutriHope® Fibre is not contraindicated for patients with hypertension, and it can be safely used in these patients under medical supervision and criteria.
References:
- Basile J, Kaplan NM. Overview of hypertension in adults. 2014;(Jnc 7):1-45.
- Kaplan, Norman M, Forman, John P. Diet in the treatment and prevention of hypertension. UpToDate. 2015;(Ldl):1-11.
https://medilib.ir/uptodate/show/3852
https://www.who.int/news-room/fact-sheets/detail/hypertension
Can NutriHope® Fibre be provided for small bowel feeding?
Yes, NutriHope Fibre is suitable for small bowel feeding depending on the patient’s state of health and care conditions.
To know moreThe term enteral nutrition is used to comprise all forms of nutritional support that imply the use of ‘‘dietary foods for special medical purposes’’ independent of the route of application. It includes oral nutritional supplements (ONS) as well as tube feeding via nasogastric, nasoenteral or percutaneous tubes.
When the patient is unwilling or unable to maintain adequate nutrition with oral feeds alone, some considerations must be taken to decide upon the type of access and site of administration of the enteral formula. There are several ways to deliver enteral nutrition:

Gastric feeding is the most common route for tube feeding and has a number of advantages to other routes: it is more physiological, easier to begin, access is more convenient and usually patients tolerate intragastric feeding well.
However, passing a feeding tube beyond the pylorus, providing the formula directly to the duodenum or jejunum, known as small bowel feeding, may be necessary in children and adults who are intolerant of intragastric feeds. This is most often due to a combination of gastroesophageal reflux, poor gastric motility and/or concerns about pulmonary aspiration, patients with disorders of gastric or oesophageal anatomy, or other reasons (ex. recurrent emesis, post-surgery/multiple trauma, etc.).
In children, jejunal feeds have traditionally been with elemental or hydrolysed formulas, and less viscous formulas due to the narrow volumen of tubes needed to pass the pylorus, although polymeric feeds have also been tolerated.
In adults, polymeric formulas are usually used except for those with malabsorptive disorders (e.g. patients with advanced intestinal or hepatobiliary failure, severe acute pancreatitis etc.) or other clinical conditions for which semi-elemental or elemental formulas may be warranted. Therefore, beyond specific indications, whole protein, complex carbohydrate feeds can be infused into the jejunum at low volumes, although sometimes, to achieve caloric requirements hydrolysed protein formulas with medium-chain triglycerides and corn syrup/glucose polymer as sources of fat and carbohydrate respectively may be required.
The sudden arrival of a hyperosmolar feed is likely to lead to abdominal cramping, hyperperistalsis, and diarrhoea since the jejunum relies on a controlled delivery of isotonic substrate. In addition, the small bowel cannot expand its capacity as does the stomach, so jejunal feeds should be administered continuously by pump and never by bolus.
The osmolarity of NutriHope Fibre High Energy formula (1,5 Kcal/ml) is 576 mOsm/litre and 446 mOsm/litre for the Moderate Energy (1,25 Kcal/ml) formula. The prescriber can decide for each patient which dilution he prefers depending on patient’s condition and tolerance. Other factors as infusion rate and the regime planned for the patient (“starting low and advancing slow”) can play a role in patients’ tolerance to small bowel feeding and will be decided based on medical assessment and criteria. NutriHope® Fibre is lactose and gluten free, making it suitable for small bowel feeding when other conditions also exist.
References:
- Zarling EJ, Parmar JR, Mobarhan S, Clapper M. Effect of enteral formula infusion rate, osmolality, and chemical composition upon clinical tolerance and carbohydrate absorption in normal subjects. J Parenter Enter Nutr. 1986;10(6):588-595. doi:10.1177/0148607186010006588
- Duggan C. Enteral feeding: Gastric versus postpyloric. 2016:1-14.
- Parrish CR, Copland AP. Enteral nutrition in the adult short bowel patient: A potential path to central line freedom. Pract Gastroenterol. 2021;45(4):36-51.
- Chapple LAS, Kouw IWK, Summers MJ, et al. Muscle Protein Synthesis after Protein Administration in Critical Illness. Am J Respir Crit Care Med. 2022;206(6):740-749. doi:10.1164/rccm.202112-2780OC
- Doley J. Enteral Nutrition Overview. Nutrients. 2022;14(11). doi:10.3390/nu14112180
- Zadák Z, Kent-Smith L. Basics in clinical nutrition: Commercially prepared formulas. e-SPEN. 2009;4(5):212-215. doi:10.1016/j.eclnm.2009.05.005
Can NutriHope® Fibre be provided to persons with diabetes?
Yes, NutriHope® Fibre can be used for tube feeding for diabetic patients with glucose monitoring and diabetic management.
To know moreDuring acute illness, the principles of nutritional support of the diabetic are no different from those for the non-diabetic but should always take into account the metabolic problems peculiar to diabetes and be accompanied by bedside glucose monitoring.
For patients with diabetes receiving enteral feeds, who require insulin for glucose control, different insulin schemes can be adopted depending on type and amount of enteral formula, feeding schedule (continuous, intermittent, bolus etc.), other comorbidities and factors that will influence glucose homeostasis. Therefore, the doctor will decide on the insulin scheme that best suits the patient’s status. Whatever scheme is chosen, health workers must be aware that if the enteral feeds (continuous or bolus) are unexpectedly discontinued, an intravenous 10 percent dextrose solution (or other equivalent dextrose solution), providing a similar number of carbohydrate calories to that which was being administered via the enteral feeds, should be infused in order to prevent hypoglycaemia.
Many of the non-diabetic formulae can be used in the diabetic, provided that the level of blood glucose is monitored and controlled appropriately. The administration of an excess of rapidly absorbed carbohydrate at too fast a rate should be avoided. A polymeric feed of moderate carbohydrate content, administered slowly by continuous infusion, may reduce this problem1. The inclusion of fiber in the formula could facilitate glycemic management by delaying gastric emptying and the intestinal absorption of carbohydrate2.
It should be recalled that the stress response to illness or injury causes insulin resistance so that insulin may be needed at higher doses than normal in insulin-dependent diabetic patients. Also, both hyperglycemia and hypoglycemia are associated with adverse outcomes in patients with diabetes mellitus as well as nondiabetic patients.
Alternative formulas, with lower carbohydrate and higher monounsaturated fat content, have been developed for diabetic patients. These formulas may be useful in patients requiring long-term home enteral nutrition. They have been evaluated in these patients on glycemic or lipid control and showed a better blood glucose control3. However, they have limited application to hospitalized patients requiring enteral nutrition as they were performed in long-term care facilities, rehabilitation, or other outpatient settings and were not sufficiently powered to detect differences in morbidity and/or mortality4.
References:
- Dardai, E. Basics in clinical nutrition: Nutritional support in the diabetic patient. E-SPEN Eur. E-J. Clin. Nutr. Metab. 4, e304–e307 (2009).
- Elia, M. et al. Enteral Nutritional Support and Use of Diabetes-Specific Formulas for Patients With Diabetes. DIABETES CARE 28, (2005).
- Ojo, O., Weldon, S. M., Thompson, T., Crockett, R. & Wang, X.-H. The Effect of Diabetes-Specific Enteral Nutrition Formula on Cardiometabolic Parameters in Patients with Type 2 Diabetes: A Systematic Review and Meta–Analysis of Randomised Controlled Trials. Nutrients 11, 1905 (2019).
- McMahon, M. M. et al. A.S.P.E.N. Clinical Guidelines: Nutrition Support of Adult Patients With Hyperglycemia. J. Parenter. Enter. Nutr. 37, 23–36 (2013).
Can pregnant and lactating women receive NutriHope® Fibre? How much?
Yes, NutriHope® fiber can be consumed by pregnant and lactating women. There is no risk of exceeding tolerable upper intake levels (UL) for micronutrients for a daily consumption up to 2800 kcal. The protein requirements of pregnant and lactating women may be met from consuming 1500 kcal. Vitamins and elements needs may not be fully covered at this level of consumption.
To know moreReferences:
Dietary Reference Intakes for Calcium, Phosphorous, Magnesium, Vitamin D, and Fluoride (1997); Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline (1998); Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids (2000); Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc (2001); Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate (2005); Dietary Reference Intakes for Calcium and Vitamin D (2011); and Dietary Reference Intakes for Sodium and Potassium (2019). These reports may be accessed via www.nap.edu.
Can NutriHope® Fibre be given to adolescents 13 years old and beyond?
NutriHope® Fibre is a nutritionally complete hyperproteic formula, with fibre, lactose and gluten free. It has specifically been designed for the use in adult patients (> 18 years) at risk or with disease-related malnutrition, therefore its nutrient content is suitable for this purpose. One litre of the moderate energy formula (1250 kcal) meets an adult’s average daily requirements of vitamins, minerals and trace elements.
If a decision is made to use NutriHope Fibre for adolescents, this a medical decision on a case-by-case basis, based on medical criteria and under the responsibility of the prescriber. In every case, other considerations such as fluid intake, renal solute load, vitamin and minerals requirements, and adequate monitoring ought to be taken into account to monitor the adequacy of individual dietary nutrient and fluid intakes.
To know moreSelection of a formula for enteral feeding depends on the age, weight, and degree of maturity of the patient, as well as his or her gastrointestinal, renal, and other metabolic functions. Other considerations include whether the patient has dietary protein sensitivities or carbohydrate or fat malabsorption, the severity of any underlying illness and other factors.
For the adolescents age group, all these aspects must be considered to select the appropriate enteral formula for the patient. Currently, no specific formula for this age group exists, and for children 10 or 13 years of age and older, formulas designed for adults usually can be used. Standard adult enteral formulas are available for children older than 10 to 13 years. The major concern surrounds adolescent patients with markedly elevated energy and protein requirements attributable to severe malnutrition, trauma, burn injuries or other reasons. Although high-energy, high-nitrogen, hypertonic formulations are well tolerated by adults with elevated metabolic needs, they may not be tolerated by these patients. Adolescents with high requirements may be managed better with high-energy density paediatric formulations (1.5 kcal/mL) rather than adult ones.
Can NutriHope® Fibre be given to children less than 13 years old?
NutriHope® Fibre is a nutritionally complete hyperproteic formula, with fibre, lactose and gluten free. It has specifically been designed for the use in adult patients (> 18 years) at risk or with disease-related malnutrition, therefore its nutrient content is suitable for this purpose. One litre of the moderate energy formula (1250 kcal) meets an adult’s average daily requirements of vitamins, minerals and trace elements.
Where, resulting from a medical assessment and subsequent decision, an adult formula is going to be provided to a child, particular care is required to ensure that the child’s fluid requirements are met (in addition to the amount of formula provided). Furthermore, vitamin and minerals intakes will need monitoring, as, amongst other things, the addition of individual nutrient supplements may be required.
To know moreWhen a child needs enteral nutrition, a decision must be made to select the appropriate enteral formula for the child. This formula must supply adequate nutrients in a form and volume that the child can tolerate. In selecting an appropriate formula, the factors to consider include the following:
- Age and medical condition
- Protein and calorie requirements
- History of food intolerance or allergy
- Intestinal function
- Route of delivery
- Formula characteristics (ex. osmolality, viscosity, nutrient content, convenience, cost etc.)
- Others
Formulas are categorized by age group of the recipient. Each is designed to be nutritionally complete if administered to the right age group and in the right quantity.
Infants under 12 months should be given breast milk, a standard infant formula, or a special infant formula.
For most children from 1 to 10-13 years of age, paediatric enteral formulas are designed specifically to meet their nutrient requirements. These formulas are complete and balanced. About 1000-1300 ml will meet 100% of the DRI for vitamins and minerals. These formulas are generally isotonic and easily tolerated by most children.
In the past, adult formulas were used for the nutritional support of children older than 1 year because paediatric formulas were not available. The primary disadvantages of using adult formulas for young children are the elevated renal solute load and insufficient vitamin and mineral levels. As well, the protein and fibre content of adult formulas are higher than a child may require.
What are the sides effects of NutriHope® Fibre?
NutriHope® Fibre does not present specific side effects of itself. However, side effects related to enteral nutrition (EN) can occur with any enteral formula provided for the nutritional support of patients at risk of or with disease related malnutrition. This can depend on several variables and many of these side effects not even be related to the enteral formula.
To know moreAlthough EN is a relatively safe procedure with proven benefits and is appropriate for patients who need it, complications can arise. Fortunately, life-threatening events are rare. However, problems with the tube, the site of administration, the rate and method of administration and/or the composition of the formula can interfere with the achievement of nutritional goals. Complications may also be the indirect result of underlying disease or medical treatment.
EN complications can be classified as mainly gastrointestinal, mechanical and metabolic. Briefly, gastrointestinal complications include diarrhoea, nausea and vomiting and/or constipation; mechanical complications include pulmonary aspiration, tube-related complications (incorrect positioning, perforation, bleeding, etc.) or tube blockage; metabolic complications include electrolyte and fluid imbalances, or refeeding syndrome, among others.
To minimize complications, prior consideration should be given to the patient’s medical and physical condition, including any metabolic or electrolyte abnormalities, previous diet, dietary tolerance, and the time that has elapsed since the last significant oral or enteral nutrition. In addition, once implemented, close monitoring of patients is an efficient safeguard against complications and side effects.
It is important to notice as well, that the presence of any of these conditions (ex. diarrhoea, nausea, electrolyte imbalance etc.), are not necessarily related to EN. There are other causes that need to be considered and ruled out. Therefore, the fact that these symptoms arise, should not make the caregivers directly assume that they are linked to EN, but rather follow the algorithms of differential diagnosis to reach to the correct one.
References:
- Stroud M, Duncan H, Nightingale J. Guidelines for enteral feeding in adult hospital patients. Published online 2003.
- Kozeniecki M, Fritzshall R. Enteral Nutrition for Adults in the Hospital Setting. Nutr Clin Pract. 2015;30(5):634-651. doi:10.1177/0884533615594012
- Ukleja A, Gilbert K, Mogensen KM, et al. Standards for Nutrition Support: Adult Hospitalized Patients. Nutr Clin Pract. 2018;33(6):906-920. doi:10.1002/ncp.10204
- Bodoky G, Kent-Smith L. Basics in clinical nutrition: Complications of enteral nutrition. e-SPEN. 2009;4(5):209-211. doi:10.1016/j.eclnm.2009.05.003
What are the contra-indications of NutriHope® Fibre?
- Not adapted for individuals who do not have the diseases, disorders or medical conditions for which the product is intended
- Not suitable for parenteral (I.V.) administration.
- Not suitable for individuals < 13 years old.
- Not suitable for patients with galactosaemia.
- Not suitable when enteral nutrition is not permitted (gastrointestinal obstruction, bowel ischemia or necrosis, etc.).
- Contraindicated if allergy and/or medical condition impede the exposure to any constituent of the product.
- Do not mix drugs or other foodstuffs with the product.
What is Galactosemia and why NutriHope Fibre is contra-indicated for these patients?
Altered metabolism of galactose caused by deficient activity of one of three enzymes results in elevated blood galactose concentration (galactosemia). Classic galactosemia, caused by complete deficiency of galactose-1-phosphate uridyl transferase (GALT), is the most common and severe type. The early signs and symptoms, such as liver dysfunction, susceptibility to infections, failure to thrive, and cataracts, can be prevented or improved by early diagnosis and treatment, but patients can still have chronic and progressive neuropsychiatric disorders.
To know moreClassic galactosemia is an autosomal-recessive disorder and occurs in approximately 1 in 60,000 livebirths. However, the reported incidence of galactosemia varies geographically from 1 in 30,000 to 40,000 in Europe to one in one million in Japan. Diagnosis requires measurement of enzyme activity in red blood cells (RBCs) and genetic testing.
Galactose is a sugar found primarily in human and bovine milk and milk products as part of the disaccharide lactose. Lactose is hydrolysed to glucose and galactose by the intestinal enzyme lactase. The galactose then is converted to glucose for use as an energy source. Free galactose also is present in some fruits and vegetables, such as tomatoes, Brussels sprouts, bananas, and apples. Altered metabolism of galactose caused by deficient enzyme activity or impaired liver function results in elevated blood galactose concentration and the condition known as galactosemia.
The main goal of long-term treatment of classic galactosemia is to minimize dietary galactose intake. Galactose should be excluded from the diet as soon as galactosemia is suspected. Minimization of dietary galactose is accomplished by excluding milk and dairy products from the diet. A dietician experienced in the treatment of genetic/innates metabolic malfunctions should provide dietary management advice.
The dietetic principle in the management of all types of galactosaemia is the elimination of all sources of galactose, including human milk, as far as possible. Dietetic management commences with provision of lactose-free preparations for infants followed by formulae/formulas with a lactose content of ≤10 mg/100 kcal. In older infants, children and adults, foods containing milk or dairy products or lactose as an ingredient must be avoided, as far as possible, so that the overall daily lactose intake will be about 25 mg/100 kcal. A precise threshold for galactose/lactose intake below which adverse effects are not elicited cannot be given.
NutriHope Fibre is lactose free. A product considered “lactose free” is not necessarily 100% free of lactose, but below an established threshold. NutriHope Fibre contains ≤ 26 mg lactose/100 kcal, therefore small amounts of lactose are still present, so if a patient with galactosemia ingests NutriHope Fibre, depending on the quantity, the amounts of lactose may be higher than what is advised and as a precise threshold for galactose/lactose intake below which adverse effects do not occur cannot be given, the product is contraindicated for the use of this patients. If NutriHope Fibre is taken or prescribed for patients with galactosemia, the prescriber and/or the patient is wholly responsible for any adverse effect suffered by the patient.
References:
- Opinion S. Scientific Opinion on lactose thresholds in lactose intolerance and galactosaemia 1. 2010;8(9):1-29. doi:10.2903/j.efsa.2010.1777.Available
- Savino P. Knowledge of Constituent Ingredients in Enteral Nutrition Formulas Can Make a Difference in Patient Response to Enteral Feeding. Nutr Clin Pract. 2018;33(1):90-98. doi:10.1177/0884533617724759
- Welling L, Bernstein LE, Berry GT, et al. International clinical guideline for the management of classical galactosemia: diagnosis, treatment, and follow-up. J Inherit Metab Dis. 2017;40(2):171-176. doi:10.1007/s10545-016-9990-5
- Lloyd J, Yerbury P, Ruszala V. Clinical features and diagnosis. Clin Pharm. 2011;3(10):323-329.
Why are there fibres in NutriHope® Fibre?
For most patients, fibres are recommended in enteral formulas. Fibres are recognized as an essential part of the normal diet. Fibres have many physiological effects which are relevant for enterally-fed patients. Fibres promote regular bowel movement, reduce the risk of constipation, and significantly reduce the incidence of diarrhea. Fibre is safe and generally well-tolerated in patients. Inclusion of a mixture of soluble and insoluble fibres is today considered to represent a more physiological approach.
Why does the water need to be boiled?
Boiling is the safest method of killing pathogenic germs, including viruses, bacteria, and parasites. Bring clean water to the boil for at least 1 minute. There is no need to boil water any longer since, as soon as this temperature is reached, all microorganisms that can affect health are eliminated. Please note: The boiling point of water can vary depending on the altitude.
Why does the water to be mixed with NutriHope® Fibre need to be cooled to at least 70 °C?
It is recommended to dilute NutriHope® Fibre powder in water cooled to at least 70 °C. Above this temperature, the micronutrients in the formula could be affected. Below this temperature, dilution is possible but may take longer and leave residues at the bottom of the container. The dispersion quality also depends on the volume to be prepared, the container and the type of utensil used for reconstitution (whisk, fork).
Why can the solution be stored at room temperature for up to 2 hours for oral feeding and 6 hours for enteral feeding?
From the time NutriHope® Fibre nutritional solution is reconstituted, the shelf life is shorter for oral use, as the risk of bacteriological contamination is greater in a container open to the air (e.g. a glass) than in a hermetically sealed bag used for enteral tube feeding.
Can drugs be mixed and administered with NutriHope® Fibre?
No, mixing drugs with NutriHope® Fibre is contraindicated and should not be undertaken.
To know moreGiven the risks for physicochemical incompatibility and instability, drugs are not to be admixed with an enteral formula. Potential drug-nutrient interactions that result from a physical, chemical, physiologic, or pathophysiologic relationship between a drug and an enteral formula are the reasons why medicines cannot be mixed with the preparation and administered simultaneously.
On the other hand, this does not mean that drugs cannot be administered orally or via the feeding tube if a patient is receiving enteral nutrition, but not at the same time. To administer drugs via tube feeding, enteral nutrition must be stopped and feeding tubes flushed before and after drug administration, following appropriate drug administration practices. For example, EN can alter drug bioavailability. The bioavailability of some drugs may benefit from administration in close proximity to enteral nutrition (EN), whereas the bioavailability of others may be significantly reduced in the same circumstances. In the latter case, administration of drug should be temporally separated from EN.
Within this framework, healthcare professionals must follow the protocols and procedures in place to ensure safe practices are adhered to relating to the preparation and enteral administration of medicines to a patient receiving support via EN.
References:
Boullata JI, Carrera AL, Harvey L, Escuro AA, Hudson L, Mays A, McGinnis C, Wessel JJ, Bajpai S, Beebe ML, Kinn TJ, Klang MG, Lord L, Martin K, Pompeii-Wolfe C, Sullivan J, Wood A, Malone A, Guenter P; ASPEN Safe Practices for Enteral Nutrition Therapy Task Force, American Society for Parenteral and Enteral Nutrition. ASPEN Safe Practices for Enteral Nutrition Therapy [Formula: see text]. JPEN J Parenter Enteral Nutr. 2017 Jan;41(1):15-103. doi: 10.1177/0148607116673053. Epub 2016 Nov 5. PMID: 27815525.
What about the registration of NutriHope® Fibre?
NutriHope® Fibre is formulated according to the standards of Food for Special Medical Purposes (FSMPs): “specially processed dietary or diet products formulated for the dietary management of patients, for use exclusively for this purpose and can only be used under medical supervision. They are intended for the exclusive or partial feeding of patients whose ability to ingest, digest, absorb or metabolize ordinary foods or certain elements contained therein is limited or impaired, or who have other special needs, of medically determined nutrients and whose treatment cannot be ensured either by simple modification of the normal diet, neither by other dietary or diet foods, nor by the combination of these two methods. »
Nevertheless, since the product is intended for export to low and middle-income countries, it is advisable to refer to international standards and consult the Codex Alimentarius.
There are no WHO guidelines on the subject to date; it is the Codex standards which apply in this context, in particular the general standard CODEX STAN 146-1985 and CODEX 180-1991 which specifically targets FSMPs for foods for special medical purposes.
Regarding importation conditions, in the absence of a derogation agreement for the benefit of an NGO or a UN agency, registration of the product is often necessary through a third-party distributor and/or local representative, to facilitate the importation of NutriHope® Fibre by the customer.
The category of product registration depends on each country. The enteral nutrition product NutriHope® Fibre, as FSMP, should generally fall into a general category of nutritional supplement.
The registration dossier contains at least: samples of finished products corresponding to the technical data sheet of the product provided to the authorities, a certificate of conformity of the distributor to the ISO 22000 standard, a technical data sheet of the product mentioning the physical characteristics, the list of ingredients, the nutritional composition, the conditions of use, the safety precautions and the storage conditions; a copy of the marketing authorization of the country of origin, the control plan of the raw materials and their certificate of analysis, the description of the manufacturing processes of the finished product, a stability study, an analysis report of the batch of finished products supplied in samples.
The Nutriset Group is in charge of carrying out this registration in the countries where its customers wish to use the product.
Can we find NutriHope® Fibre in pharmacies?
Today NutriHope® Fibre is only available on order from Nutriset France, through national and international medical NGOs, government purchasing centers and/or private wholesalers for use in hospitals. Nevertheless, the sale of the product in pharmacies to allow a purchase under medical prescription is not to be excluded.